BNCM: DELEX Critical Care Products Approved by Philippines FDA
BNCM: DELEX Critical Care Products Approved by Philippines FDA
Reno, Nevada, United States, 3rd Oct 2024 – DELEX Pharma offers a wide range of Critical Care products approved by the Philippines’ Food and Drug Administration (FDA). ICU (Intensive Care Unit) is a critical environment for patients facing severe health challenges.
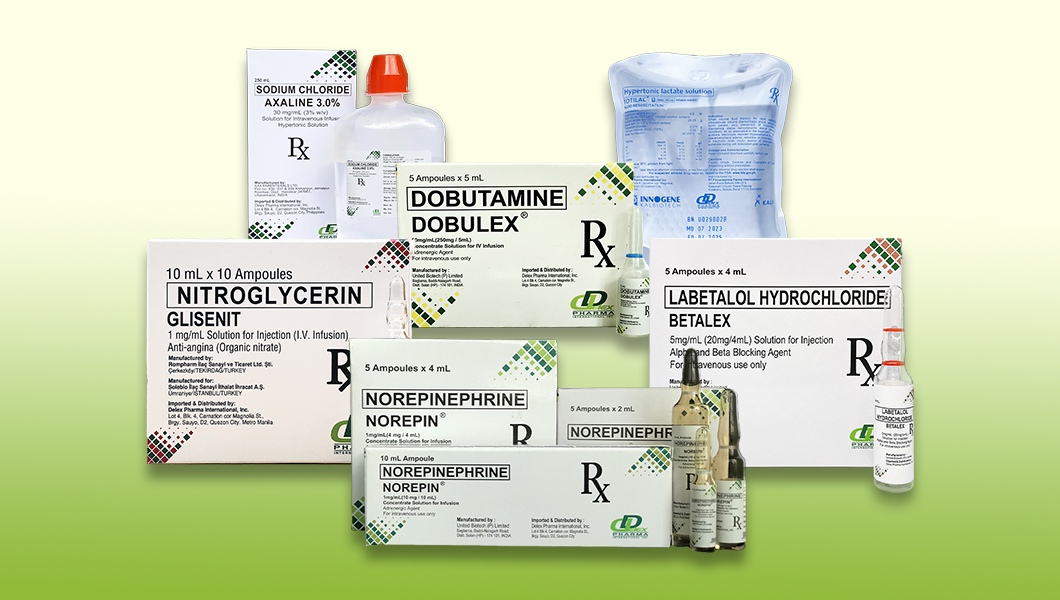
DELEX Critical Care Products
The Critical Care products are part of DELEX Pharma’s broader portfolio designed to address gaps in the availability of specialized critical care drugs. It is very essential for addressing a wide array of critical care needs, from managing severe pain and hypertension to supporting cardiac function and blood pressure.
These products are manufactured under strict compliance with FDA standards, ensuring their quality and effectiveness in critical care management. Details or specific inquiries about these products is available at DELEX Pharma website.
DELEX’s Critical Care Product Distribution
The critical care products are strategically placed in drugstore chains nationwide to enhance accessibility. One of the key distribution channels is Mercury Drug, the largest pharmacy chain in the country with thousands of branches, including those in remote areas. This extensive network significantly increases market reach and drives sales growth for the organizations critical care products.
DELEX Critical Care Product Registrations with the FDA
1.) Name of Product : GLISENIT (Nitroglycerin)
Purpose of Product : Provides relief from anginal chest pain through vasodilation
FDA Registration Number : DRP-13372
2.) Name of Product : BETALEX (Labetalol)
Purpose of Product : Treats severe hypertension, including hypertensive disorders in pregnancy
FDA Registration Number : DR-XY48744
3.) Name of Product : AXALINE 3% (Sodium Chloride)
Purpose of Product : Used in the management of severe sodium chloride depletion when electrolyte restoration is required in following cases like low salt syndrome and hyponatremia
FDA Registration Number : DR-XY48414
4.) Name of Product : DOBULEX (Dobutamine)
Purpose of Product : he product is the first-choice inotrope for patients with measured or suspected low cardiac output
FDA Registration Number : DR- XY38709
5.) Name of Product : TOTILAC (Hypertonic Lactate)
Purpose of Product : Treatment for a prolonged decrease in intracranial pressure during acute episodes of intracranial hypertension, including Traumatic Brain Injury (TBI)
FDA Registration Number : DR-XY33427
6.) Name of Product : NOREPIN (Norepinephrine)
Purpose of Product : The first-line vasopressor for hypotension due to shock
FDA Registration Number : DR-XY38818
7.) Name of Product : NICARDILEX (Nicardipine Hydrochloride)
Purpose of Product : Recommended drug for the treatment of hypertensive crisis, eclampsia, and : perioperative hypertension
FDA Registration Number : DRP-6940
About Bounce Mobile Systems, Inc. (OTC: BNCM)
BNCM is an Asset Management Company that secures its assets by investing in companies with strong growth potentials, robust revenues, significant profits, proven track records, and promising business models with highly experienced management teams that will exceptionally qualify for quotation and listing on the OTC or NASDAQ markets within the next 2 to 3 years. The growth of these companies will directly provide more employment opportunities to the communities, improve the country’s economy, and bring greater social change.
For more information, visit: https://www.bncm.net/
About Delex Healthcare Group, Inc. (DELEX)
DELEX is a Delaware-registered company, and the holding company of DLX Holdings, Inc. (DLX). DLX is a Philippine-based parent company that holds a 60% controlling interest in Delex Pharma International, Inc. an ISO 9001-2015 Certified company, which also holds a controlling interest in JMN Brother’s Pharma Limited, Inc. DLX and its subsidiaries are involved in the development and distribution of pharmaceutical and healthcare products in the Philippines.
For more information, visit: https://delexhealth.com
Media Contact
Organization: BNCM Inc.
Contact Person: Kathleen Galvez
Website: https://bncm.net
Email: Send Email
Contact Number: +17753913237
Address: 401 Ryland Street, Suite 200-A Reno, NV 89502 United States
City: Reno
State: Nevada
Country: United States
Release Id: 03102417769
